
(EPA List of Lists, 2022) CISA Chemical Facility Anti-Terrorism Standards (CFATS) No regulatory information available. & indicates that no RQ is assigned to this generic or broad class, although the class is a CERCLA hazardous substance.Glosbe utilise des cookies pour vous offrir la meilleure expérience. Cherchez des exemples de traductions antimony chloride dans des phrases, écoutez à la prononciation et apprenez la grammaire. 313c indicates that although not listed by name and CAS number, this chemical is reportable under one or more of the EPCRA section 313 chemical categories. Vérifiez les traductions antimony chloride en français.Process Safety Management of Highly Hazardous Chemicals Standard ListĮPA Consolidated List of Lists Regulatory Name Occupational Safety and Health Administration's

Cybersecurity and Infrastructure Security Agency's Chemical FacilityĪnd the U.S. Environmental Protection Agency's Title III Consolidated List of Zeitschrift für Anorganische und Allgemeine Chemie (in German). Antimony(III) chloride 99. "On the preparation and composition of the salts of antimony". In contrast to the other reagent suggested for 2-hydroxychalcones, namely antimony(V) chloride, the reaction products obtained with boron trifluoride are stable in the air and do not lose their color. : Cite journal requires |journal= ( help) "Hydrolysis of Antimony(III)-Hydrochloric Acid Solution at 25☌" (PDF).Ueber krystallisirtes Algarothpulver und Antimonoxychlorür". "On the Crystal Structure of the Antimony Oxychloride Sb4O5Cl2 and Isomorphous Oxybromide". Edstrand, Maja Brodersen, Rolf Sillén, Lars Gunnar Linnasalmi, Annikki Laukkanen, Pentti (1947)."Synthesis, properties, and hydrolysis of antimony trichloride". "The crystal structure of antimony(III) chloride oxide Sb4O5Cl2". "Chemiehistorische Experimente: Erze als Ausgangsprodukte für die Herstellung von Arzneimitteln". José Rodríguez has published a complete study devoted to the commercial network of chemical medicines developed by Vittorio Algarotti: The First Commercial Network of a Chymical Medicine:."Cyclopaedia, or, An universal dictionary of arts and sciences". pp. Préliminaire XXV, "poudre d'algaroth". Sous le privilège de l'Académie des sciences & de la Société royale de médecine. France: A Paris, chez Cuchet, libraire, rue & hôtel Serpente. Traité élémentaire de chimie, présenté dans un ordre nouveau, et d'après les découvertes modernes Avec figures Par M. Shop M-W Books Join MWU Log In Username My Words Recents. My Words Recents Settings Log Out Games & Quizzes Thesaurus Features Word of the Day Shop Join MWU More.
Antimony chloride full#
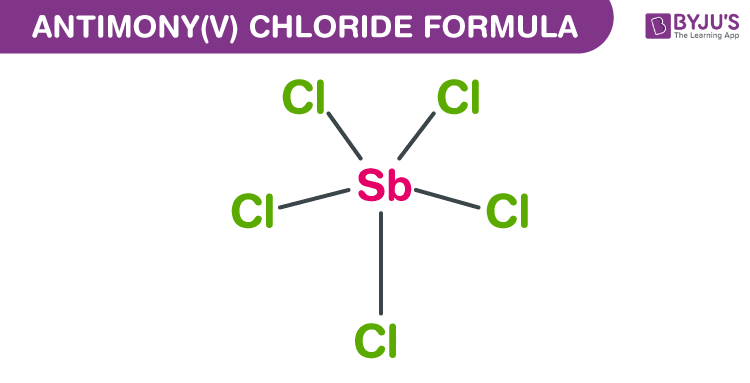
either of two chlorides of antimony: See the full definition Hello, Username. ^ Lidin, Sven Johnsson, Mats Hugonin, Zuzana (2009). The meaning of ANTIMONY CHLORIDE is either of two chlorides of antimony."The stability of onoratoite, Sb8O11Cl2, in the supergene environment". Mineralogical Magazine and Journal of the Mineralogical Society. "Onoratoite, a new antimony oxychloride, from Cetine di Cotorniano, Rosia (Siena, Italy)". Antimonous chloride Antimontrichlorid (German) Antimony butter Antimony chloride (Sb2Cl6) Antimony trichloride Antimoontrichlride (Dutch) Butter of antimony C.I. "The structures of onoratoite, Sb8O11Cl2 and Sb8O11Cl2.6H2O". "The structure of onoratoite, (, Br) revisited". ^ Mayerová, Zuzana Johnsson, Mats Lidin, Sven (2006)."Physiochemical analysis of antimony trioxide–antimony trichloride, antimony trioxide–antimony tribromide systems". SbCl 3 + H 2O → SbOCl + 2 HCl References Alternative historical names ĭissolving antimony trichloride in water yields antimony oxychloride: However, onoratoite is a known Sb-O-Cl mineral, its composition being Sb 8Cl 2O 11. Neither SbOCl nor the latter compound occur naturally. Today the hydrolysis of antimony trichloride is understood first the SbOCl oxychloride is formed which later forms Sb 4O 5Cl 2.
The suggestion of SbOCl being a mixture of antimony trichloride and antimony oxide or pure SbOCl were raised. The exact composition was unknown for a very long time. Vittorio Algarotti introduced the substance into medicine, and derivatives of his name (algarot, algoroth) were associated with this compound for many years. In 1659 Johann Rudolf Glauber gave a relatively exact chemical interpretation of the reaction. Its production was first described by Basil Valentine in Currus Triumphalis Antimonii.


 0 kommentar(er)
0 kommentar(er)
